Step-by-step explanation:
When ice melts at
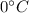 , it requires some energy in the form of heat called the latent heat of fusion.
, it requires some energy in the form of heat called the latent heat of fusion.
This is the energy required to melt the ice into the water at
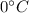 . This energy changes the state of ice from solid to liquid.
. This energy changes the state of ice from solid to liquid.
Similarly, the same amount of energy must be extracted to convert water into ice.
Although energy is provided, the temperature is not changing because that energy is used to change the state of water.