Answer:
d = 0.0625 m
Explanation:
The velocity of an object dropped from a tall building is given by :
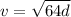
Where
d is the distance in feet
We need to find the distance when the velocity is 32 ft/s
Rearranging for d,
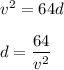
Put v = 32 ft/s
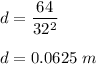
So, the required distance is 0.0625 m.