Answer:

Step-by-step explanation:
Regardless of the type of gas, 1 mole at standard temperature and pressure (STP) occupies a volume of 22.4 liters. In this case the gas is helium (He).
We can set up a ratio.
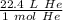
Multiply by the given number of moles.
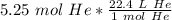
The moles of helium will cancel.
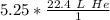
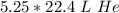
Multiply.
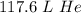
5.25 moles of helium gas at STP is 117.6 liters of helium.