Answer: 75.7 % yield
Step-by-step explanation:
The balanced chemical equation is:

According to stoichiometry :
68.2 g of
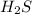 will require = 64 g of
will require = 64 g of
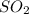
Thus 7.5 g of
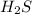 will require =
will require =
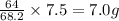 of
of
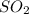
Thus is the limiting reagent as it limits the formation of product and is the excess reagent.
As 68.2 g of give = 96 g of
Thus 7.5 g of
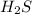 give =
give =
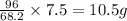 of
of
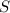
% yield=
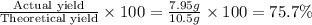
Thus 75.7 % yield is there.