Answer:
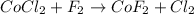
3.000 moles of flourine are required to produce 290.8 g of cobalt(II) fluoride
Step-by-step explanation:
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
When cobalt chloride is added to flourine, chlorine being more reactive than flourine, displaces chlorine atom its salt solution and lead to formation of cobalt flouride and chlorine gas.
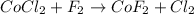
To calculate the number of moles, we use the equation:
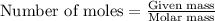
According to stoichiometry :
1 mole of
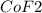 require = 1 mole of
require = 1 mole of
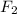
Thus 3.000 moles of
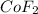 will require=
will require=
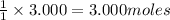 of
of
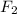
Thus 3.000 moles of flourine are required to produce 290.8 g of cobalt(II) fluoride