Answer:
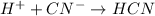 , product favoured
, product favoured
Step-by-step explanation:
Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and present in an aqueous are represented in the form of ions.
Net ionic equation : In the net ionic equations, we do not include the spectator ions in the equations.
When hydrochloric acid react with potassium cyanide, then it gives potassium chloride and hydrocyanic acid as products.
The complete ionic equation will be:
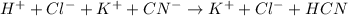
The net ionic equation will not contain spectator ions which are
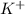 and
and
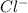 :
:
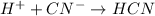
The reaction is product favoured.