Answer:
(b) both the temperature and pressure of the gas decrease.
Step-by-step explanation:
An ideal gas undergoes an adiabatic expansion, a process in which no heat flows into or out of the gas. As a result, both the temperature and pressure of the gas decrease.
Gay Lussac states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
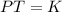
Also, according to the first law of thermodynamics which states that energy cannot be created or destroyed but can only be transformed from one form to another. Thus, the ideal gas does work on the environment with respect to the volume and temperature.