Answer:
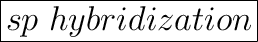
Step-by-step explanation:
In HCN,
Carbon is singly - bonded ( 1 sigma bond ) to Hydrogen while triply - bonded ( 1 sigma, 2 pi bonds) to Nitrogen.
Thus, the Stearic No. for Carbons is:
= No. of sigma bonds + No. of lone pairs
= 2 + 0
= 2
According to Stearic No.:
=> sp = 2
=> sp² = 3
=> sp³ = 4
Hence,
The hybridization of C in HCN is sp.
![\rule[225]{225}{2}](https://img.qammunity.org/2022/formulas/mathematics/high-school/3icqlwn6du2l5ygbr7z2lp6sjjralcpq09.png)
Hope this helped!
~AH1807