Answer:
![[Ag^+]=2.82x10^(-4)M](https://img.qammunity.org/2022/formulas/chemistry/college/zhbcds0rtcxlz9s6upvq4ud80clinpkmh5.png)
Step-by-step explanation:
Hello there!
In this case, for the ionization of silver iodide we have:
![AgI(s)\rightleftharpoons Ag^+(aq)+I^-(aq)\\\\Ksp=[Ag^+][I^-]](https://img.qammunity.org/2022/formulas/chemistry/college/pdu18sz1vk8oznqj8pr7i3tyrugdgt0rpd.png)
Now, since we have the effect of iodide ions from the HI, it is possible to compute that concentration as that of the hydrogen ions equals that of the iodide ones:
![[I^-]=[H^+]=10^(-3.55)=2.82x10^(-4)M](https://img.qammunity.org/2022/formulas/chemistry/college/4lewb1b3kafy52t81ipfdpymvkn8nhj4xj.png)
Now, we can set up the equilibrium expression as shown below:
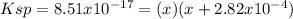
Thus, by solving for x which stands for the concentration of both silver and iodide ions at equilibrium, we have:
![x=[Ag^+]=2.82x10^(-4)M](https://img.qammunity.org/2022/formulas/chemistry/college/vwezxhew8o1dz8uohumv7l68v7hc3ihg2a.png)
Best regards!