Answer:
New pressure = 7.51 atm
Step-by-step explanation:
Given that,
Initial pressure, P₁ = 4.5 atm
Initial temperature, T₁ = 100°C = 100+273 = 373 K
Final temperature, T₂ = 350°C = 350 + 273 = 623 K
We need to find the new pressure. Using the below relation as follows :
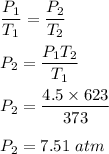
So, the new pressure is 7.51 atm.