Answer:

Step-by-step explanation:
First, we must convert molecules to moles.
We use Avogadro's Number: 6.022*10²³. This number tells us the amount of particles (atoms, molecules, etc.) in 1 mole of a substance. In this case, it is molecules of F₂
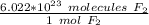
Multiply by the given number of molecules.
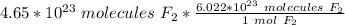
Flip the fraction so the molecules of fluorine cancel.
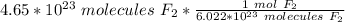
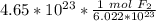
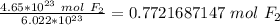
Next, convert the moles to liters. Assuming this is at STP (standard temperature and pressure), there are 22.4 liters in 1 mole of any gas.
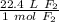
Multiply by the number of moles we calculated.
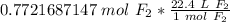
The moles of fluorine cancel.
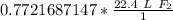
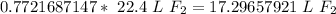
The original measurement has 3 significant figures (4, 6, and 5), so our answer must have the same. For the number we calculated, that is the tenth place. The 9 in the hundredth place tells us to round the 2 up to a 3.
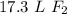
There are approximately 17.3 liters of fluorine.