Answer:
22.21 grams of aluminum (Al) was produced in the reaction if 30.00 grams of magnesium (Mg) was consumed.
Step-by-step explanation:
The balanced reaction is:
2 AlPO₄ (aq) + 3 Mg (s) → 2 Al (s) + 1 Mg₃(PO₄)₂ (aq)
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
- AlPO₄: 2 moles
- Mg: 3 moles
- Al: 2 moles
- Mg₃(PO₄)₂: 1 mole
Being the molar mass of each compound:
- AlPO₄: 122 g/mole
- Mg: 24.31 g/mole
- Al: 27 g/mole
- Mg₃(PO₄)₂: 262.93 g/mole
By reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
- AlPO₄: 2 moles* 122 g/mole= 244 grams
- Mg: 3 moles* 24.31 g/mole= 72.93 grams
- Al: 2 moles* 27 g/mole= 54 grams
- Mg₃(PO₄)₂: 1 mole* 262.93 g/mole= 262.93 grams
You can apply the following rule of three: if by stoichiometry 72.93 grams of Mg produces 54 grams of Al, 30 grams of Mg produces how much mass of Al?
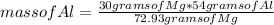
mass of Al= 22.21 grams
22.21 grams of aluminum (Al) was produced in the reaction if 30.00 grams of magnesium (Mg) was consumed.