Answer:
C) 0.800 mol
Step-by-step explanation:
In order to convert from moles of Al₂O₃ into moles of Al, we'll need to use the stoichiometric coefficients, using a conversion factor that has Al₂O₃ moles in the denominator and Al moles in the numerator:
- 0.400 mol Al₂O₃ *
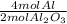 = 0.800 mol Al
= 0.800 mol Al
So the correct answer is option C).