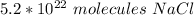Answer:

Step-by-step explanation:
1. Convert grams to moles
First, convert grams to moles using the molar mass. This can be found on the Periodic Table.
- Na: 22.9897693 g/mol
- Cl: 35.45 g/mol
Sodium (Na) has an oxidation state of +1 and chlorine (Cl) has an oxidation state of -1, so they combine in a 1:1 ratio for a formula of NaCl. We can simply add their moles masses.
- NaCl: 22.9897693 g/mol + 35.45 g/mol = 58.4397693 g/mol
Use this as a ratio.
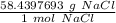
Multiply by the given number of grams.
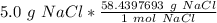
Flip the fraction so the grams of sodium chloride cancel.
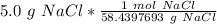
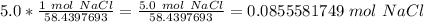
2. Convert moles to molecules
We must use Avogadro's Number. This tells us the amount of particles (molecules, atoms, etc.) in 1 mole of a substance. In this case, it is molecules of sodium chloride.
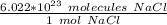
Multiply by the number of moles we calculated.
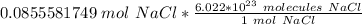
The moles of sodium chloride cancel.
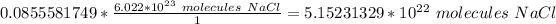
The original measurement of grams has 2 (2 and 0) significant figures, so our answer must have the same. For the number we calculated, that is the hundredth place. The 5 in the hundredth place tells us to round the 1 to a 2.