Answer: a. 249.5 g/mol
b. 1.996 g
c. Measure 1.996 g of
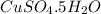 and dissolve in water until the volume is 400 ml
and dissolve in water until the volume is 400 ml
Step-by-step explanation:
Molar mass is the sum of atomic masses of all the elements present.
Thus molar mass of
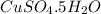 = 1(63.5)+1(32)+4(16)+5(18) = 249.5 g/mol
= 1(63.5)+1(32)+4(16)+5(18) = 249.5 g/mol
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
To calculate the number of moles for given molarity, we use the equation:
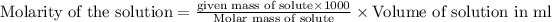
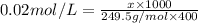
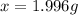
c. We need to measure 1.996 g of
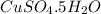 and dissolve it in water to make the volume 400 ml.
and dissolve it in water to make the volume 400 ml.