Answer: 126.1 grams of are needed to make 5.3 L of 0.25 M solution
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
To calculate the mass of solute for given molarity, we use the equation:
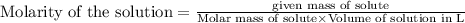
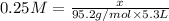
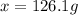
Thus 126.1 grams of are needed to make 5.3 L of 0.25 M solution