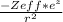Answer:
Answer is explained in the explanation section below.
Step-by-step explanation:
Penetration refers to the proximity of electrons in an orbital to the nucleus.
Deeper-penetrating electrons have less shielding and therefore a higher Effective nuclear charge (Zeff), but they better shield other electrons.
To illustrate penetration, we may use the idea of Zeff, or effective nuclear charge. It's actually the difference between the number of charged protons and the number of shielded electrons. To put it another way, how effective the nucleus is at attracting electrons. Since they do not shield themselves, the core electrons penetrate the most and are exposed to the most strong nuclear charge.
The electron probability density is highest in the orbital's centre or nucleus for 28-orbitals.
In a multi-electron unit, the electron density near the nucleus of an atom for each shell and subshell of an electron is used to measure the nucleus penetration by an electron.
Since it has a higher electron density near the nucleus, the 2s electron penetrates the nucleus of the atom more than the 2p electron.
A 2s electron is less well shielded by the core electrons than a 2p electron because it can spend more time near the nucleus as a result of the penetration.
Mathematical Expression:
v =