Answer:
1.25 grams of chlorine.
Step-by-step explanation:
Hello there!
In this case, since it is possible to define the parts per million of chlorine as the milligrams of chlorine per liters of water, in order to obtain the mass of chlorine in 2500 L, we proceed as follows:
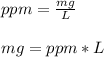
In such a way, we plug in the given 0.500 ppm and 2500 L to obtain (in grams):
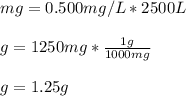
Best regards!