Answer: A. 2.00 moles
Step-by-step explanation:
To determine the number of moles of nickel in a 117 g Ni sample, we can divide the mass of the sample by the molar mass of nickel:
moles = mass ÷ molar mass
= 117 g ÷ 58.69 g/mol
= 2 moles
Therefore, there are approximately 2 moles of nickel in a 117 g Ni sample and the correct answer is A. 2.00 moles
It is important to note that the molar mass of a substance is defined as the mass of one mole of that substance. One mole of a substance is equal to
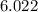 ×
×
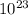 units of that substance, which is known as Avogadro's number. The molar mass of a substance is often used to convert between mass and number of moles, as shown in the calculation above.
units of that substance, which is known as Avogadro's number. The molar mass of a substance is often used to convert between mass and number of moles, as shown in the calculation above.