Answer:
1.8 moles per liter of solution per second.
Step-by-step explanation:
The rate at which carbon dioxide is being formed in this reaction can be calculated by using the balanced chemical equation for the combustion of propane:
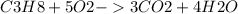
From the balanced chemical equation, it is clear that for every mole of propane consumed, 3 moles of carbon dioxide are formed. Therefore, if the rate of consumption of propane is 0.6 mol/L s, the rate of formation of carbon dioxide is 3 * 0.6 = 1.8 mol/L s.
This means that the rate of formation of carbon dioxide is 1.8 moles per liter of solution per second.