Answer:
0.898 moles
Step-by-step explanation:
In order to solve this, we need the gas constant:
gas constant: R=0.082057338 L atm K^(-1) mol^(-1)
p = pressure = 1.34 atm
v = 15.4 L
t = temperature = 280K
pv / (Rt) =
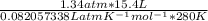 = ==> substitute known values
= ==> substitute known values
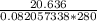 = ==> simplify
= ==> simplify
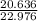 =0.898 moles
=0.898 moles