Answer:
Choice b:
![4\sqrt[6]{x^5}](https://img.qammunity.org/2023/formulas/mathematics/high-school/nnzxra41tfuryuuu79hfvabjsj2lj5f9it.png)
Explanation:
First of all we can eliminate a and c because the 4 appears under the root sign but it should be outside
Next we have to determine what
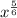 actually means
actually means
This is the same as
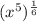
![(x^5)^{(1)/(6)} = \sqrt[6]{x^5}](https://img.qammunity.org/2023/formulas/mathematics/high-school/93h5l0s3cko4grxoc65bpc2h98q5mz1vdz.png)
x raised to the power 1/6 means that it is the 6th root of the x
So
![(x^5)^{(1)/(6)} = \sqrt[6]{x^5}](https://img.qammunity.org/2023/formulas/mathematics/high-school/93h5l0s3cko4grxoc65bpc2h98q5mz1vdz.png)
Together with the multiplier of 4 we get
![4x^{(5)/(6)} = 4\sqrt[6]{x^5}](https://img.qammunity.org/2023/formulas/mathematics/high-school/udot1p7o0x0yg0yl2lp1tlqw7n4tfqgdvg.png)
Answer is choice b