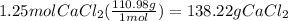Answer:
138.22 g
Step-by-step explanation:
First, determine the molecular weight of
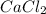 :
:
M.W. Ca = 40.08
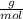
M.W. Cl = 35.45
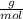
So,
M.W.
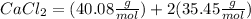
M.W.
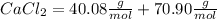
M.W.
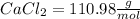
Recall that one mole of anything is equal to
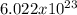 of that thing (as one dozen is equal to 12).
of that thing (as one dozen is equal to 12).
So,
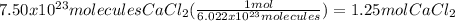
Next, determine the mass: