We are given –
- Energy of photon is = 3 eV
To find the wavelength of photon we have to use the Planck Expression.

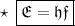

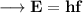

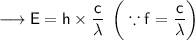
Where, E is the energy of photon and f is the frequency of photon & h's called Planck Constant.

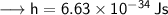
 Speed of light, c =
Speed of light, c =
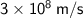
⠀⠀⠀━━━━━━━━━━━━━━━━━━━━━━━━━━


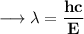

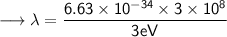

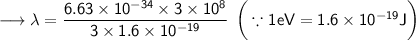

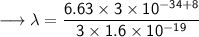

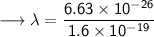

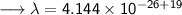

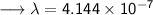

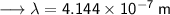

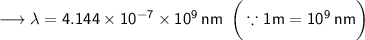

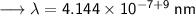

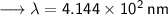

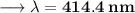
- Henceforth, wavelength will be 414.4nm.