Answer:
p=2.74 atm
Step-by-step explanation:
According to Boyle's Law, at a constant temperature, a gas' pressure is inversely proportional to volume. This relationship is used to establish the following equation:
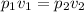
For this problem, let
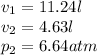
So,
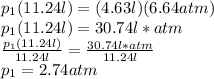
This answer makes sense according to Boyle's Law. As the volume decreases, pressure must increase. Since the initial volume is higher, the initial pressure should be lower.