Answer:
A.) 5.17 atm
Step-by-step explanation:
According to the Ideal Gas Law, PV=nRT where:
P= pressure in atm
V= volume in L
n= moles of gas
R= ideal gas constant
T= temperature in K
To calculate moles of propane, first calculate the molecular weight:
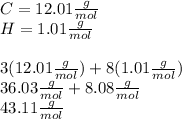
Then use that molecular weight to convert g to mol:
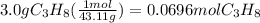
So, for this problem, let:
V=0.325 L
n=0.0696 mol
R=0.08205
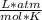
T=(28+273)K=301K
Next, rewrite the Ideal Gas Law to solve for P:
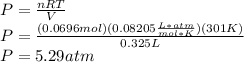
The result isn't exactly any of the answers, but I assume there was a difference in rounding somewhere. The only answer that is even remotely close is the 5.17 atm.