Answer:

Step-by-step explanation:
To find the molarity of the sodium hydroxide solution, we first need to consider the balanced chemical equation for the reaction between sodium hydroxide (NaOH) and sulfuric acid (H2SO4):

From the balanced equation, we can see that 1 mole of sulfuric acid reacts with 2 moles of sodium hydroxide. Given that 22.30 mL of 0.253 M sulfuric acid reacts exactly with 28.7 mL of the sodium hydroxide solution, we can calculate the moles of sulfuric acid:
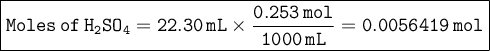
Since 1 mole of
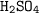 reacts with 2 moles of NaOH, we can find the moles of NaOH:
reacts with 2 moles of NaOH, we can find the moles of NaOH:

Now, we can calculate the molarity of the sodium hydroxide solution:

So, the molarity of the sodium hydroxide solution is approximately 0.393 M.
#BTH1
________________________________________________________