Answer:
13.04 %
Step-by-step explanation:
First, find the Molecular mass of the individual elements present in the compound.
Carbon = 12
Hydrogen = 1
Oxygen = 16
Then, find the total mass of C₂H₅OH
(12 × 2) + (1 × 5) + 16 + 1 = 46
In order to find the percentage mass of hydrogen, you must take the Molecular mass of hydrogen in the compound, divide it by the Molecular mass of the compound and times that by 100.
% by mass of hydrogen =
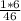 × 100
× 100
[it is 1 × 6 because 1 is the mass of hydrogen and there are 6 hydrogen atoms in C₂H₅OH]
% by mass of hydrogen = 13.04 %