Here, We are asked to calculate the molarity of a solution with 18.4 moles of Lithium Fluoride in 26 kg of water... To find the molarity, We need to divide the number of moles by the volume of solution in liters
»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»«»
- Number of moles = 18.4
- Volume of solution = 26 kg = 37.14 liters
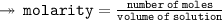
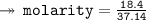
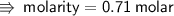
➪ Therefore, The molarity of solution is 0.71 molar...~