Answer:
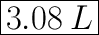
Step-by-step explanation:
The volume of the heated balloon can be found by using the Charles' law formula which is;
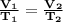
where
T1 is the initial temperature
T2 is the final temperature
V1 is the initial volume
V2 is the final volume
From the question
T1 = 323K
T2 = 398K
V1 = 2.5 L V2 = ?
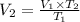
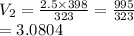
We have the final answer as
3.08 L