Answer:
The substance's half-life is 6.1 days.
Explanation:
The half-life of the substance can be calculated by knowing the constant decay:
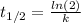
k: is the decay constant = 0.1133 d⁻¹
Hence, the half-life is:
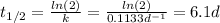
Therefore, the substance's half-life is 6.1 days.
I hope it helps you!