When electrons move from a trajectory with a low energy level to a higher energy level, it absorbs energy called excitation.
At a certain stationary level electrons only have a certain level of energy anyway. The level of such energy is formulated.
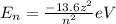
where Z denotes an atomic normor, and n denotes a particular shell or a certain quantum number of an electron's stationary trajectory.
2s is on second layer, so n = 2 and Z for Na is 11
Based on thus formula, energy for 2s electron is 1223 because we need higher energy to move from based layer than others