
#1
We can solve through ammonia or oxygen .Lets go through ammonia
- 4mol of ammonia produces 2mol NO
- 2mol of ammonia produces 1mol NO
- 1mol of ammonia produces 0.5mol NO.
Moles of Ammonia
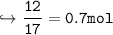
Moles of NO
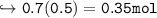
Mass of NO
#2
NH_3 is excess reagent and O_2 is limiting reagent .
#3
We need ∆n