Answer:
[H⁺] = 2.57x10⁻⁶ M
Step-by-step explanation:
This problem can be solved using the definition of pH:
pH = -log[H⁺]
Now we isolate [H⁺] in the equation:
-pH = log[H⁺]
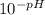 =[H⁺]
=[H⁺]
As we are given the pH by the problem, we can now proceed to calculate the [H⁺]:
[H⁺] =
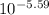
[H⁺] = 2.57x10⁻⁶ M
Thus, when the pH of a solution is 5,59; the molar concentration of H⁺ species is 2.57x10⁻⁶.