Answer: The newly occupied volume is 11.46 L
Step-by-step explanation:
Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
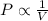 (At constant temperature and number of moles)
(At constant temperature and number of moles)
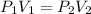
where,
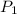 = initial pressure of gas = 0.999 atm
= initial pressure of gas = 0.999 atm
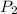 = final pressure of gas = 0.802 atm
= final pressure of gas = 0.802 atm
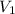 = initial volume of gas =
= initial volume of gas =
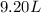
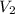 = final volume of gas = ?
= final volume of gas = ?
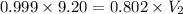
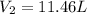
Therefore, the newly occupied volume is 11.46 L