Answer:
The sample of __Ca___ contains the greatest number of atoms
Step-by-step explanation:
As we know,
1 mole
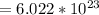 molecular entities (Can be atom, ions or molecules)
molecular entities (Can be atom, ions or molecules)
Number of atoms in 1.76 mole sample of Be
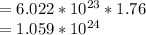
The sample of __Ca___ contains the greatest number of atoms