Answer:
58.53 L
Step-by-step explanation:
Ideal gas equation is
PV = nRT
P - the pressure of the gas = 104.5 KPa
V- the volume occupied by the gas
n - the number of moles of gas =2.50
R - the universal gas constant, usually given as 0.0821 atm.L/(mol K)
T - the absolute temperature of the gas.= 298K
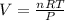
Now substituting vales we get
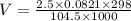
V = 58.53 L