Answer:
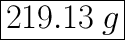
Step-by-step explanation:
To find the mass of the substance given it's density and volume, we use the formula;
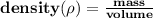
Since the mass is the unknown variable in the question, we make mass the subject and solve for it.
mass = density × volume
From the question
- density = 0.8765 g/ml
- volume = 250 ml
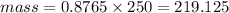
We have the final answer as
219.13 g