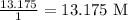Answer: 13.175
Step-by-step explanation:
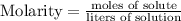
To find the moles of solute, we begin by finding the formula mass of NaOH.
- The atomic mass of Na is 23 g/mol.
- The atomic mass of O is 16 g/mol.
- The atomic mass of H is 1 g/mol.
- So, the atomic mass of NaOH is 40 g/mol.
This means 527 grams of NaOH is equal to
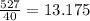 moles.
moles.
So, the molarity is