Answer: 672.2 grams
Step-by-step explanation:
With 564.1 kJ of heat energy,
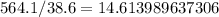 moles of ethanol can be combusted.
moles of ethanol can be combusted.
So, we need to find the formula mass of ethanol.
- The atomic mass of carbon is 12 g/mol.
- The atomic mass of oxygen is 16 g/mol.
- The atomic mass of hydrogen is 1 g/mol.
- So, the formula mass of ethanol is
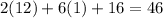 g/mol.
g/mol.
Therefore, the mass of ethanol is
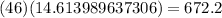 grams.
grams.