Answer:
HCO₂
Step-by-step explanation:
From the information given:
The mass of the elements are:
Carbon C = 26.7 g; Hydrogen H = 2.24 g Oxygen O = 71.1 g
To determine the empirical formula;
First thing is to find the numbers of moles of each atom.
For Carbon:
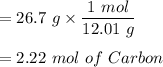
For Hydrogen:
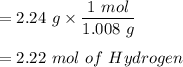
For Oxygen:
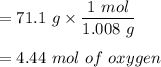
Now; we use the smallest no of moles to divide the respective moles from above.
For carbon:
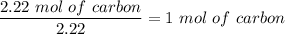
For Hydrogen:
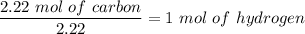
For Oxygen:
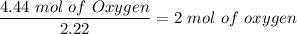
Thus, the empirical formula is HCO₂