Answer:
D. heat flows into the system and its internal energy increas
Step-by-step explanation:
The question defines the system as the piece of gold. The answer will depend on the gold's perspective of what is happening. If we assume room temperature to be cooler than
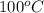 , then the gold will experience energy coming in until the tempeartures of both the water and gold are the same. As heat flows into the gold, its internal energy will increase. Option D seems to address both processes correctly.
, then the gold will experience energy coming in until the tempeartures of both the water and gold are the same. As heat flows into the gold, its internal energy will increase. Option D seems to address both processes correctly.