First, lets examine the formula for that of Ammonia.
Ammonia = NH3
Notice that in the formula, there are 3 hydrogen atoms in a single ammonia molecule. This being said, if there are 200 molecules of ammonia in the given scenario, multiply 200 by 3.
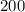 ×
×
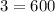 ← Amount of (H) Atoms Present in 200 (NH3) Molecules
← Amount of (H) Atoms Present in 200 (NH3) Molecules
Hope this helps!