Answer:
n = 0.0102 mol.
Step-by-step explanation:
Hello there!
In this case, according to the ideal gas law, which allows us to set up a relationship among volume, pressure, temperature and moles, we can define it as:
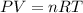
Thus, given the volume in liters (0.1000 L), temperature in kelvins (298.15 K) and pressure in atmospheres (2.50 atm), we can solve for moles as shown below:
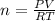
Thereafter, we plug in the aforementioned values to obtain:
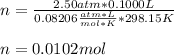
Best regards!