Answer:
pH = 13.7.
Step-by-step explanation:
Hello there!
In this case, as we set up the chemical reaction between nitric acid and sodium hydroxide:
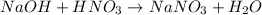
It is possible to realize there is a 1:1 mole ratio of acid to base, thus, we next compute the moles of each one:
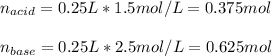
In such a way, since the base react with more moles, there is leftover that we compute as shown below:
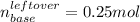
Afterwards, we compute the concentration given the new volume of 500 mL (0.500 L), as both volumes are added up:
![[base]=0.25mol/0.500L=0.5M](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/22860swvss4lbpicggkbj6.png)
Now, since sodium hydroxide is such a strong base, we compute the pOH first:
![[OH^-]=[base]=0.5M](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/zdxvwyqkrpn1bdt1z1019g.png)
![pOH=-log([OH^-])=-log(0.5M)\\\\pOH=0.30](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/9rs0hpi0zm1uczjwgiuwse.png)
And the pH:
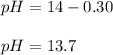
Best regards!