Answer:
0.752 L (3 s.f.)
Step-by-step explanation:
A cylinder with a moveable piston contains 1.20 L of a gas at 45.0 atm and 314 K. The pressure and temperature increase to 96.0 atm and 420 K.
To find the new volume of the cylinder, we can use the combined gas law.
Combined Gas Law
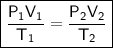
where:
- P₁ is the initial pressure.
- V₁ is the initial volume.
- T₁ is the initial temperature (in kelvin).
- P₂ is the final pressure.
- V₂ is the final volume.
- T₂ is the final temperature (in kelvin).
The values to substitute into the formula are:
- P₁ = 45.0 atm
- V₁ = 1.20 L
- T₁ = 314 K
- P₂ = 96.0 atm
- T₂ = 420 K
Rearrange the formula to isolate V₂:
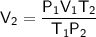
Substitute the values into the formula and solve for V₂:
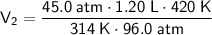
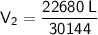
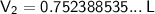
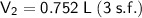
Therefore, the new volume of the cylinder is 0.752 L (3 s.f.).