Rank these elements according to first ionization energy (highest to lowest) : Ne, F, O, N, C, B, Be, Li. HINT: There is a general trend for ionization energy within a period of the periodic table. However, elements with a filled

or half-filled
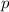
subshell have a higher ionization energy than expected by the general trend.