Chemical links
Atoms bond to reach a lower energy state. That is to say, the atoms that are loose are not stable and for this reason they unite forming the compounds and in this way they gain stability.
A chemical bond occurs when atoms share electrons.
A covalent bond is a force that joins two atoms of non-metals.
If you have Br_{2}
Atoms of the same element participate and have a bond.
Given its electronic configuration:
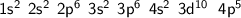
Adding the electrons of the energy sublevel indicates that bromine (Br) has 7 electrons in its last energy shell.
And that it lacks an electron to comply with the OCTET RULE (this indicates that incomplete atoms try to complete the electrons by forming chemical bonds).
But when joining both would be sharing an electron, thus completing its octet. Through this transfer of electrons where one of the atoms gives up electrons and the other accepts them, to have the same electronic configuration of the closest noble gas in the periodic table (in this case to Krypton).
The Alpha.