Answer:
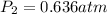
Step-by-step explanation:
According to Boyle's Law:
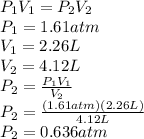
This makes sense because according to Boyle's Law, the volume of a given amount of gas at a constant temperature is inversely proportional to its pressure. As the volume increases, the pressure must decrease proportionately.