Answer:
0.8832 atm
Step-by-step explanation:
Per Boyle's Law:
If the temperature is held constant, the volume of a fixed amount of gas is inversely proportional to its pressure:
V∝
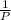
and
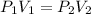
To find pressure resulting from a change in volume:
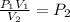
So:
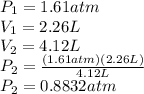
This answer makes sense because Boyle's Law states that a given volume of gas at a constant temperature should have a proportionally lower pressure as the volume is increased.